"If the [ice] crystal is exceedingly small, rather than growing, it may melt."
SUPERCOOLED CLOUDS 1
Summary
Several aspect of icing clouds physics are discussed.
Key Points
- Supercooling and nucleation rates are key to the formation of icing clouds.
- Vapor pressure differences of liquid water and ice play a role.
- Tips are given for recognizing supercooled clouds.
- Types of icing clouds are discussed.
Discussion
This includes a theoretical understanding of the physics of icing clouds, but also practical tips, such as how to identify supercooled icing clouds in flight.
While this is not a long lecture (11 pages), I left out of this review for brevity items such as the artificial seeding of clouds, which is only tangentially related to aircraft icing, although I will briefly discuss that here.
Wikipedia summarizes about the author and presenter Bernard Vonnegut:
Bernard Vonnegut (August 29, 1914 – April 25, 1997) was an American atmospheric scientist credited with discovering that silver iodide could be used effectively in cloud seeding to produce snow and rain. He was the older brother of American novelist Kurt Vonnegut.
His brother, Kurt Vonnegut, alluded to Bernard's work in some of his works, most famously in Cat's Cradle.
The discovery about silver iodide seeding is dated as 1946.
Vonnegut is credited with hundreds of publications before 1953 2 (and many more after)
so Vonnegut was well known in 1953 when he presented this lecture.
Some may recall that a form of ice "ice-nine" was a science fiction plot element in the novel Cat's Cradle 3, but there will be no spoilers here.
"There are several ways," Dr. Breed said to me, "in which certain liquids can crystallize - can freeze - several ways in which their atoms can stack and lock in an orderly, rigid way."
(One can only imagine the conversations the brothers had to inspire "ice-nine". There is a real form of ice, Ice IX, but it does not have the properties of ice-nine in the novel.)
Curiously, Irving Langmuir (of whom we have seen much of in this website, such as in Mathematical Investigation of Water Droplet Trajectories) is sometimes credited with the discovery about silver iodide. However, Langmuir's own writing credits Vonnegut. Langmuir and Vonnegut at times worked together at the GE research labs. Langmuir later conducted much research into cloud seeding. You can learn more of Langmuir's and Vonnegut's collaborations at 4.
On to the lecture, which begins with "Ice Clouds and Supercooled Clouds" (it has no other introductory material).
Ice Clouds and Supercooled Clouds
Two different types of clouds are possible when the temperature of the atmosphere is below the melting point of ice, ice crystal clouds, and supercooled clouds. Ice crystal clouds are a suspension of tiny ice crystals, usually hexagonal plates or columns, that are so small that they fall very slowly. Supercooled clouds are a suspension of tiny water droplets which remain frozen even though the temperature may be far below the melting point of ice. Supercooled clouds are almost exactly like the usual water clouds that exist above 0°C., except for the fact that, because their temperature is below the melting point of ice, they are unstable.
Why Liquids Supercool and Homogeneous Nucleation
At first it may seem anomalous that liquid water drops can remain unfrozen even at low temperatures, for one generally thinks that water freezes at 0°C. However, experiments show that water and practically all liquids supercool, some of them to over one hundred degrees below their melting point. Water has been cooled to about -39°C (1 , 2) without freezing and mercury (3) and tin (5) to 50 degrees and 110 degrees Centigrade below their melting points.
A supercooled liquid is unstable. If a crystal of the same composition is introduced into a supercooled liquid;,the crystal grows at the expense of the liquid so long as the system remains below the melting point of the crystal.
The above statement concerning the ability of a crystal to grow in a supercooled liquid of the same composition is generally true, but it needs some qualification. If the crystal is exceedingly small, rather than growing, it may melt. This follows from the well-established physical fact that very small particles have higher free energies than the same material in bulk (5).
When the crystals are of the order of 0.1 micron or less, the free energy of the surface becomes appreciably relative to the bulk free energy. Therefore, if the crystal is sufficiently small, it can have a free energy greater than that of the supercooled liquid. As a consequence, it will melt rather than grow, even though the liquid is far below the melting point of a large crystal. For any given temperature of the supercooled liquid, a crystal must be larger than a certain critical size in order to grow. The lower the temperature, the smaller will be this critical size.
If we consider a clean supercooled liquid, free of any foreign particles, the freezing process cannot begin, until somehow, by chance collisions of the liquid molecules, a small crystal of the critical size or larger is formed6. The lower the temperature, the smaller is the critical size, so that the likelihood of spontaneous freezing increases very rapidly with decreasing temperature. With the exception of viscous liquids, there is usually a limit to the extent to which a liquid can be supercooled. The above process of freezing, in which crystallization starts in a clean supercooled liquid by the chance formation of a minute crystal, is known as spontaneous or homogeneous nucleation.
Heterogeneous Nucleation and Aircraft Icing
An airplane, flying through a supercooled cloud, collides with the supercooled cloud droplets and becomes wet with a thin continuous layer of supercooled water. The abrupt collision of the droplets with the surface of the airplane probably aids in the nucleation of ice formation and the area of the airplane surface is so large that even at very moderate supercooling, the chances of ice crystal formation are very large. The ice crystals grow very rapidly through the supercooled water on the surface and, in a matter of seconds, the surface is covered with ice. From then on, the supercooled droplets, landing on the surface of ice, cause it to grow in thickness.
It is possible at temperatures very close to the melting point that supercooled water can exist on the surface of an airplane for an appreciable time before freezing, but this is probably quite unlikely. In considering the transformation of supercooled water to ice, it should be pointed out that the 80 calories of heat released by the freezing of each gram of water has a very considerable heating effect. Unless this heat is removed, it warms the water up to the melting temperature and only a fraction of the water freezes. On the surface of a moving airplane, this heat is rapidly dissipated, so that the freezing process continues until the water is all frozen. If the temperature of the air is only slightly below 0°C, this heat-transfer process can limit the rate of freezing so that the water runs back on the wing somewhat, before it freezes.
At this point, it is worthwhile pointing out that clouds composed of ice crystals rather than supercooled water drops do not cause ice formation on airplanes. When an airplane flies through a cloud of small ice crystals, they do not adhere to it, but merely bounce off, producing no ill effects other than giving the plane an electrostatic charge which can be troublesome to radio operation.
Vapor Pressure Instability of Supercooled Clouds
All clouds composed of particles suspended in the atmosphere are unstable. In the first place, they are gravitationally unstable and slowly fall to the ground. In the second place, they have a very large surface which gives them an energy greater than that of bulk material. For this reason, they want to form bigger and fewer particles. In addition to these two kinds of instability, a supercooled cloud is unstable for still another reason. Supercooled water has a higher free energy than ice and therefore has a higher vapor pressure than ice at the same temperature. Figure 1 is a graph drawn to an arbitrary scale showing the relationship of the vapor pressure of ice to that of supercooled water. The vapor-pressure curve of supercooled water is a smooth extension of the curve for water above freezing. The curve for ice is a separate curve that always lies below that of supercooled water. A very important consequence of this difference in vapor pressure between ice and supercooled water is that supercooled water droplets rapidly evaporate and condense on any ice crystals which may be present. If, somehow, ice crystals are formed or are introduced in appreciable numbers into a supercooled cloud they rapidly grow and the supercooled cloud rapidly vanishes. As we will see, this vapor pressure difference between supercooled water and ice is of fundamental importance in understanding the behavior of supercooled clouds.
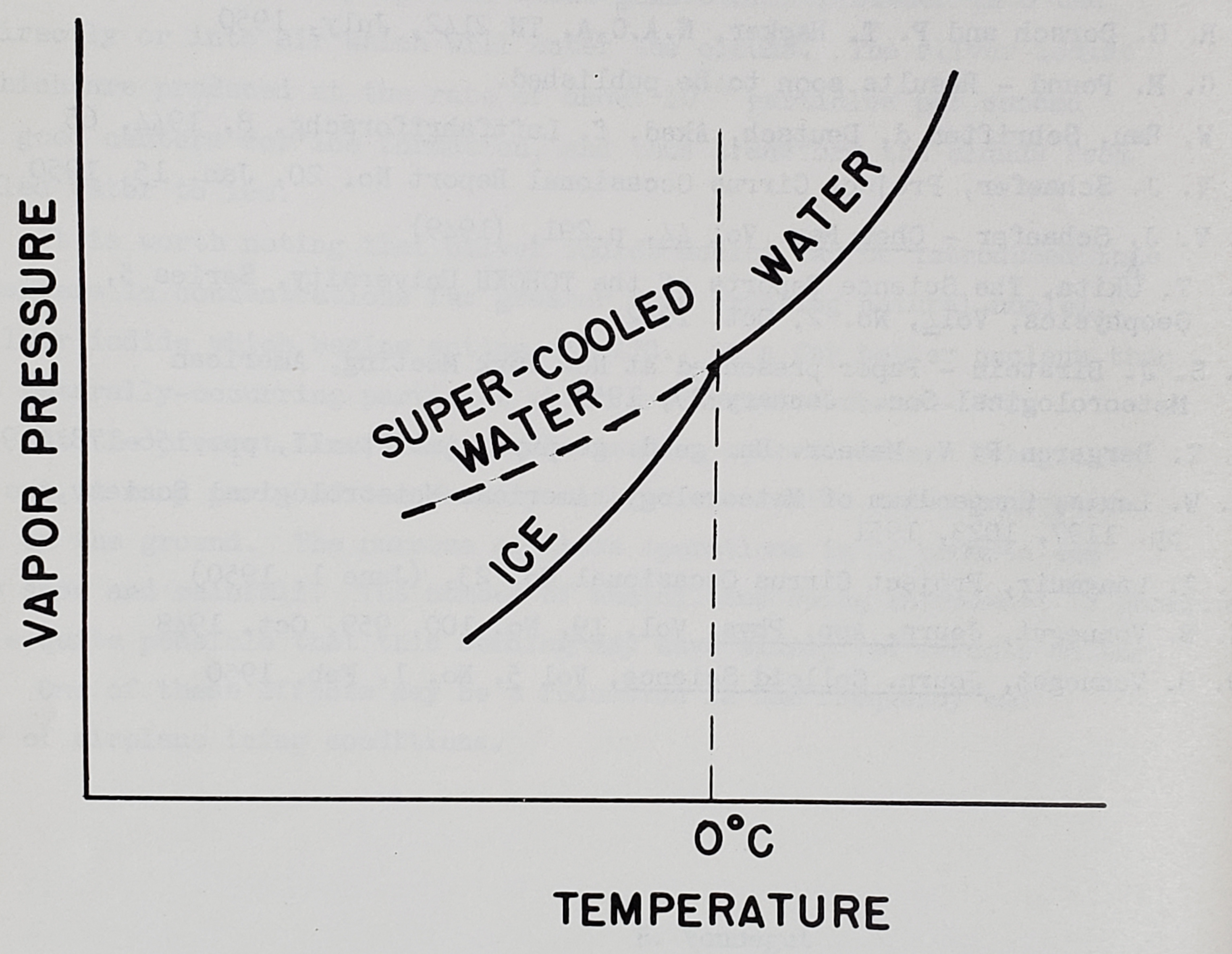
Formation and Dissipation of Supercooled Clouds
In the light of the foregoing background we can now proceed to the consideration of the formation and behavior of supercooled clouds in the atmosphere. If no ice crystals form, we can expect that supercooled clouds would behave in very much the same way as clouds above zero degrees Centigrade. As can be seen from Figure 1, the vapor-pressure curve of supercooled water is a smooth continuation of the curve for water. It is true that because the vapor pressure decreases with decreasing temperature, we might expect that diffusion processes and evaporation processes would be slower than at higher temperatures. Other than this, there is no reason for thinking supercooled clouds would be markedly different.
The striking feature of supercooled clouds that distinguishes them is their instability with respect to ice. A knowledge of the ice forming process is, therefore, essential to an understanding of supercooled clouds. It is developing that the ice-forming process in the atmosphere is sometimes a process of homogeneous nucleation without foreign particles playing much of a role, and at other times a process of heterogeneous process with air-borne particles playing a big part. First, let us consider the simplest case, when foreign particles are not important. Following the researches of Gulling 1 and Schaefer 2, there is now a growing, but by no means universal, agreement that even in the cleanest atmosphere which can be produced in the laboratory, supercooled water clouds cannot be formed or exist at temperatures below about -40°C. These observations made in the normal atmosphere and in specially purified atmospheres indicate that at -40°C. the rate of spontaneous nucleation becomes enormously rapid and supercooled water cannot exist.
Recognition of Ice Clouds and Supercooled Clouds
Supercooled clouds look very much the same as ordinary non-supercooled clouds. They have the same appearance and assume the same wide variety of shapes and sizes. From its appearance alone, it is impossible to tell whether or not a cloud is supercooled. However, if by measurement or estimate it is known that the temperature of the cloud is below freezing, it is frequently possible to tell by its appearance whether or not it is composed of supercooled water drops. If the cloud is thin enough so that the sun can be seen through it, the edge of the sun will usually appear to be quite diffuse and hazy if the cloud is composed of ice crystals. On the other hand, if the sun appears as a disc with sharply defined edges, the cloud is probably liquid water droplets. This effect is caused by the fact that the particle size in ice-crystal clouds is usually somewhat larger than in water-drop clouds. This method of distinguishing between the two types of clouds breaks down if very large water drops or very small ice crystals are present.
A much more foolproof method for ascertaining that a cloud is made of ice particles rather than water drops is the observation of halos or sections of halos at 22° from the sun. This phenomenon is caused only by ice crystals. Another phenomenon that is caused only by ice crystals is the so-called sun pillar, a bright, vertical line of light passing through the sun.
This effect is caused by reflections from the horizontal surfaces of ice crystals. When a cloud layer of ice crystals is observed from above, the sun pillar frequently is quite striking and closely resembles the appearance of the sun or moon reflected in the ripples of a lake. The observation of a sun pillar on clouds is good evidence that they are composed of ice crystals. On the other hand, if no sun pillar is observed and if the shadow of the airplane on the clouds is surrounded with colored rings, sometimes called "glory", it is probable that the cloud is composed of supercooled water.
Characteristics of Various Types of Supercooled Clouds
With the possible exception of Cirrus clouds, which are generally composed of ice crystals formed at high altitudes and low temperatures, all of the conventional cloud types can occur in the supercooled condition. The important variables which determine the severity of an icing condition, liquid water content, drop size distribution, and temperature, vary widely in super-cooled clouds, as they do in clouds below the freezing level.
The most severe icing conditions are encountered in strongly convective cumulus clouds in which warm, moist air is rapidly raised to high altitudes and low temperature. Lewis (16) has calculated a theoretical maximum liquid-water content in such clouds of the order of 2.5 grams per cubic meter and reports a maximum observed value of about 1.8 grams per cubic meter. Fortunately, aircraft are usually able to avoid flying through such clouds. It is also fortunate that the frequency of occurrence of such high liquid-water content in cumulus clouds is often limited by the formation of ice crystals, which rapidly convert the supercooled water to snow. The intense activity of cumulus clouds probably aids the formation of ice crystals by the chain reaction resulting from crystal fragmentation. In addition, cumulus clouds frequently extend to high altitudes where ice crystal formation from nuclei and spontaneous nucleation is accelerated by the low temperature.
Stratus-type clouds are frequently supercooled, but usually they have much lower liquid water content than cumulus and seldom exceed one gram per cubic meter, usually having only a few tenths of a gram per cubic meter. This type of cloud formation can present a serious icing hazard because it frequently extends in an unbroken mass for hundreds of miles. Ice crystals generally do not form as readily in supercooled stratus clouds as they do in cumulus. This is probably because there is less convective activity and because stratus clouds usually do not reach the high altitudes and low temperatures that cumulus clouds attain. For this reason, stratus icing conditions can sometimes exist over wide areas for many hours without dissipating as snow. The measurements made by Lewis (16) and others show that the drop diameter in both stratus and cumulus type supercooled clouds ranges from a few microns to about 30 microns with cumulus clouds on the average having drops a little larger than those in stratus.
Conclusions
A few of the observations are dated, but in general this lecture holds up well after 70 years.
If you want up-to-date information, the University of Kansas "Aircraft Icing: Meteorology, Protective Systems, Instrumentation and Certification" 5 course offers much more information in this vein. This course has been offered for decades (I think it was 1993 when I attended), and has been a "rite of passage" for many of us working in aircraft icing (as I imagine the University of Michigan Airplane Icing Information Course may have been circa 1953).
Citations
I did not find citations of this lecture.
As we saw above, Vonnegut's works has been cited hundreds of times, and this short publication may have received little notice beyond the attendees of the Airplane Icing Information Course.
Vonnegut cites 19 references, reference 8 is a NACA publication:
- Dorsch, Robert G., and Hacker, Paul T.: Photomicrographic Investigation of Spontaneous Freezing Temperatures of Supercooled Water Droplets. NACA-TN-2142, 1950. ntrs.nasa.gov
There is reference 16 by William Lewis, who wrote several of the NACA publications:
- Lewis, William: Compendium of Meteorology, American Meteorological Society, pp. 1197, 1023, 1951.
And reference 17 by Irving Langmuir:
- Langmuir, Irving: Project Cirrus Occasional No. 23, June 1, 1950.
Notes
-
Vonnegut, Bernard: "Supercooled Clouds" Lecture No. 1 in University of Michigan, College of Engineering: Airplane icing information course. Lectures presented in a special program at the University of Michigan, Ann Arbor, March 30-April 3, 1953. lib.umich.edu ↩
-
Vonnegut, Kurt: "Cat's Cradle", Holt, Rinehart and Winston, 1963. ↩
-
Langmuir Laboratory for Atmospheric Research, Storms Above the Desert, langmuir.nmt.edu ↩
-
Course detail: Aircraft Icing: Meteorology, Protective Systems, Instrumentation and Certification, www.enrole.com ↩